Magic mushrooms
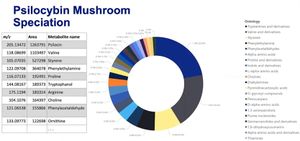
Magic mushrooms, sometimes referred to as psilocybin mushrooms are entheogens which have been used for spiritual, religious, and divinatory purposes around the world. Whilst these mushrooms are renowned for containing the psychedelic chemical psilocybin (a tryptamine) like all phytomedicines they also contain an array of parts of psilocybin and other related psychedelic compounds including phenylethylamines[1] (See Figure 1).
History
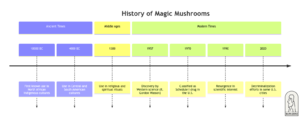
Based on depictions in rock drawings, some historians speculate that magic mushrooms may have been utilised as early as 9000 B.C. by primitive societies in North Africa (see Figure 2). More concisely they have been portrayed in the Pre-Columbian sculptures and glyphs found throughout North, Central, and South America and may also be observed in Stone Age rock art in Africa and Europe.
Species
Magic mushrooms include the biological genera Copelandia, Gymnopilus, Inocybe, Panaeolus, Pholiotina, Pluteus, and Psilocybe.
Dry Powder Storage
A common concentration of freshly homogenized P. cubensis mushroom powder is:
- 0.01 wt.% norbaeocystin
- <0.01 wt.% aeruginascin
- 0.07 wt.% baeocystin
- 1.51 wt.% psilocybin
- 0.04 wt.% psilocin.
In storage, overtime, the most pronounced changes in the decay in concentration over time were observed with psilocybin, which is the main tryptamine alkaloid in fruiting bodies.[2]
After 1 week - The concentration of psilocybin reduced from 1.51 wt.% to 1.31 wt.%. The greatest loss of psilocybin was found in a sample stored in the light at 20°C, where the psilocybin value dropped to 0.96 wt.%. This trend was also observed for norbaeocystin, aeruginascin, baeocystin, and psilocin, where the measured concentration gradually decreased. It is apparent that tryptamines are not very stable in the homogenized mushroom powder.
After 1 month - The degradation to approximately 50 % of the initial concentration of all tryptamines occurred on storage under all of the test conditions. The most significant decrease in concentration to 0.72 wt.% appeared in the sample that was stored in the light at 20°C. This effect is probably due to the photooxidation of alkaloids. The lowest concentration reduction to 0.85 wt.% was found in the dark at 20°C.
After 2 months - This phenomenon was also observed when the most significant degradation to 0.67 wt.% was measured in a sample that was stored in the light at 20°C. In contrast, the smallest loss of psilocybin occurred when the sample was stored in the dark at 20°C, where the psilocybin was 0.82 wt.%.
After 15 months - A very similar concentration of all tryptamines was found after 15 months under all of the storage conditions. The psilocybin concentration varied by a maximum of 0.04 % in these samples. The lowest degradation of psilocybin was found in a sample that was stored in the dark at 20°C. The concentration of all analytes gradually decreased, which corresponds to studies that claim the same but do not state under what conditions.
For the minor alkaloids (aeruginascin, baeocystin, norbaeocystin, and psilocin) the changes in concentrations were negligible after the first week. After one month of storage, a slight decrease in the concentration of all of the remaining alkaloids was seen at a similar time under all of the defined conditions. After two months of storage, all of the alkaloids were reduced, except for psilocin, which decomposed more only in the light at 20°C. After 15 months of storage, no further changes occurred, except for psilocin, whose concentration This article is protected by copyright. All rights reserved. increased (probably due to the degradation of psilocybin).
References
- ↑ Presence of phenylethylamine in hallucinogenic Psilocybe mushroom: possible role in adverse reactions. O Beck , A Helander, C Karlson-Stiber, N Stephansson. DOI: 10.1093/jat/22.1.45. Accessed on 18 Jan 2023 via https://pubmed.ncbi.nlm.nih.gov/9491968/
- ↑ Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Gotvaldová, K., Hájková, K., Borovička, J., Jurok, R., Cihlářová, P., & Kuchař, M. (2020). Drug Testing and Analysis, 13(2), 439–446. doi:10.1002/dta.2950